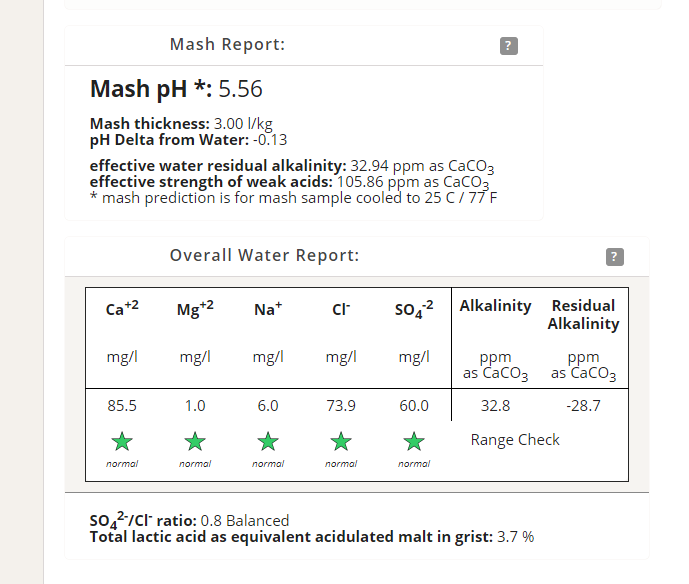
Ah! I get it now! Low bicarbonate, who cares? Are you selecting a target style? If so, it's just telling you that, in this case, you're way below what the target calls for. I generally never concern myself with target water. So here's how you get around that number, if it's bothering you: Add your bicarbonate to the kettle. Controlling the mash pH is why we do the initial load of salts and acids. I don't even worry about the sparge any more, a bit of metabisulfite to get rid of the chlorine and in it goes. My salts and acids to control the mash go into the first strike water. Bicarbonate doesn't really affect the mash that much once you've overcome the alkalinity, which is what is important to us. Your priorities when it comes to water are first, chlorine. Second, enough calcium (50 ppm or more). Third, chloride to sulfate ratio according to desired outcome (malty vs bitter), although that can be adjusted in the kettle, the enzymes don't care. Lastly, getting the water "to style." Again, this can be done in the kettle or even at bottling. About the only way I see you could bring your bicarbonate up to style without throwing other ions out of balance is to use potassium bicarbonate, available as a wine treatment to reduce acidity.