- Joined
- May 12, 2018
- Messages
- 814
- Reaction score
- 825
- Points
- 93
So I got my Chico tap water report from Ward
pH - 7.8
Cations - 2.9
Anions - 2.9
Sodium - 12
Magnesium - 14
Total Hardness, CaCo3 - 113
Sulfate, So4-S - 1
Bicarbonate, HCO3 - 156
Total Alkalinity, CaCO3 - 129
I downloaded the Brun Water spreadsheet. I have actually tried several but this one seems the most comprehensive. I also tried the BF calculator. Not settled on one yet. I'm trying to figure out how the BF calculator relates to a recipe . . . I can't figure out the pH and why it doesn't agree with the Brun Water calculator and to be honest with all the complaints I'm not sure I want to until things settle...
Right now I'm working on my Pale Ale and an "Amber Balanced" target per the Brun Water calculator
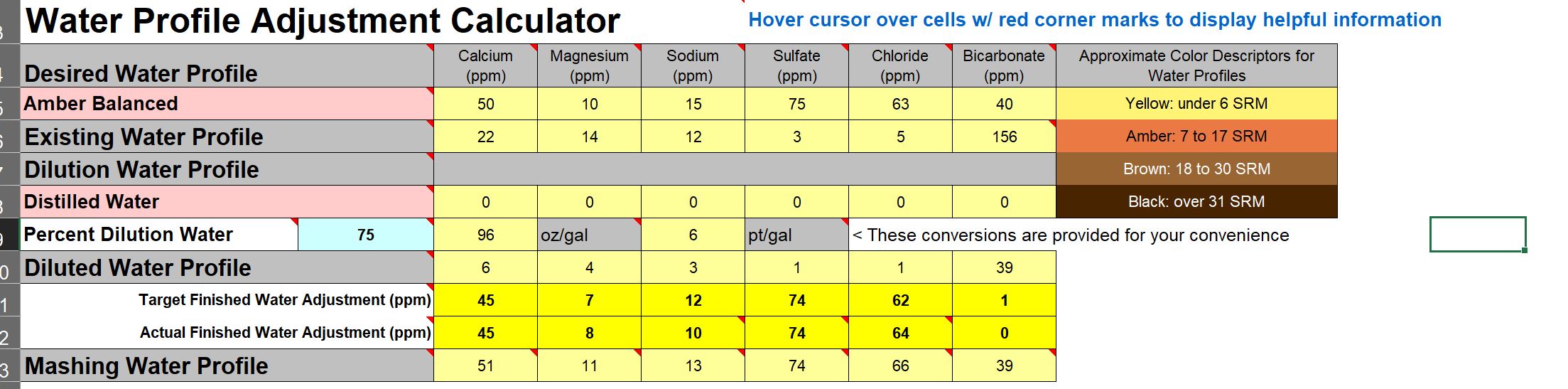
I'm guessing "balanced" means balanced SO4/Cl?
To get my HCO3 to 40 I found I need to dilute with DI water at 75% DI 25% tap and add salts (see below)
The calculator gives two kinds of water - Mash and Sparge
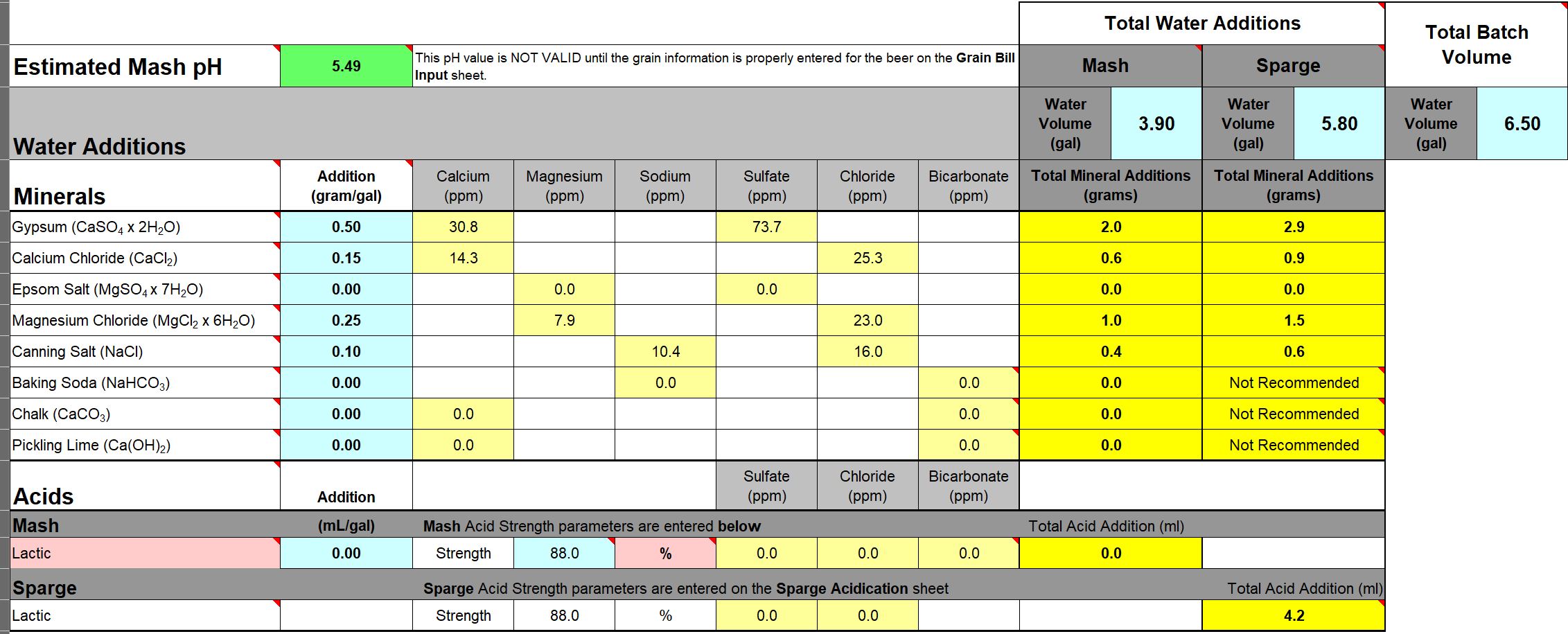
Process question - Do I add salts directly to the mash or to the water before the mash?
Target question - does the target look like a good one for a West Coast American Pale Ale?
I'm going to try this on my next House Pale Ale
I can't get the pH to align with the Brun Water calculator and I DKW but I'm not in the mood to struggle with it with the state of the site
pH - 7.8
Cations - 2.9
Anions - 2.9
Sodium - 12
Potassium - 2
Calcium - 22Magnesium - 14
Total Hardness, CaCo3 - 113
Sulfate, So4-S - 1
Chloride - 5
Carbonate, CO3 < 1.0Bicarbonate, HCO3 - 156
Total Alkalinity, CaCO3 - 129
I downloaded the Brun Water spreadsheet. I have actually tried several but this one seems the most comprehensive. I also tried the BF calculator. Not settled on one yet. I'm trying to figure out how the BF calculator relates to a recipe . . . I can't figure out the pH and why it doesn't agree with the Brun Water calculator and to be honest with all the complaints I'm not sure I want to until things settle...
Right now I'm working on my Pale Ale and an "Amber Balanced" target per the Brun Water calculator
I'm guessing "balanced" means balanced SO4/Cl?
To get my HCO3 to 40 I found I need to dilute with DI water at 75% DI 25% tap and add salts (see below)
The calculator gives two kinds of water - Mash and Sparge
Process question - Do I add salts directly to the mash or to the water before the mash?
Target question - does the target look like a good one for a West Coast American Pale Ale?
I'm going to try this on my next House Pale Ale
I can't get the pH to align with the Brun Water calculator and I DKW but I'm not in the mood to struggle with it with the state of the site
Last edited:









