- Joined
- Dec 15, 2020
- Messages
- 3
- Reaction score
- 0
- Points
- 1
Hi. Apologies for the long post before hand. I wanted to share as much data that may help narrow down the issue.
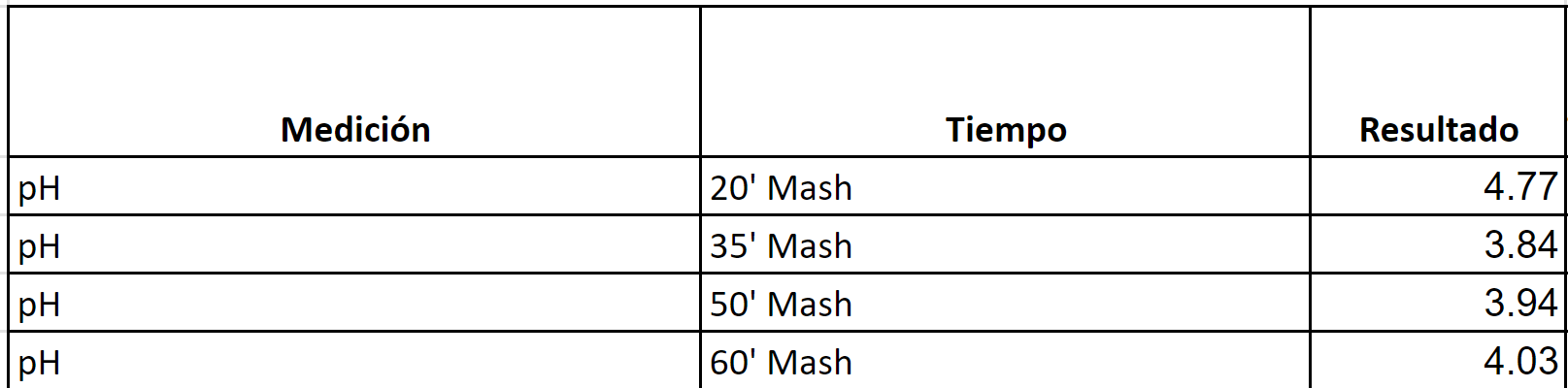
I will begin by stating that other than not hitting the FG in most of these beers by about 5 points (which could be caused by a number of variables), the beers have all tasted good. I just don't understand why the Ph readings are so far off. Below are different beers, target Ph per BrunWater and actual readings.
I appreciate any insight, what could be the cause, how can I get more consistent in hitting the 5.2-5.4 range.
Thank you!!
Batch Sizes ~30 liters
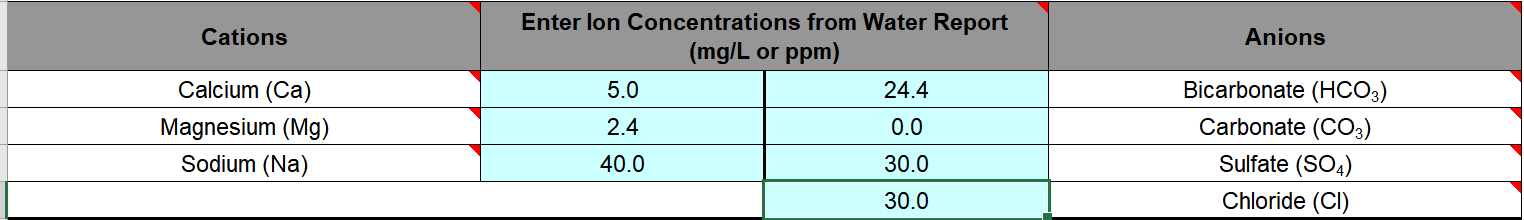
Water Profile Ph 6.9-7.1
Samples are collected at the indicated times, readings usually take place during the boil, about 60 mins after when all samples have cooled down. I've include a picture of the PhMeter, not a high quality device, but when reading the control substance a chemist friend prepares, it is within 0.1 from the control figure.
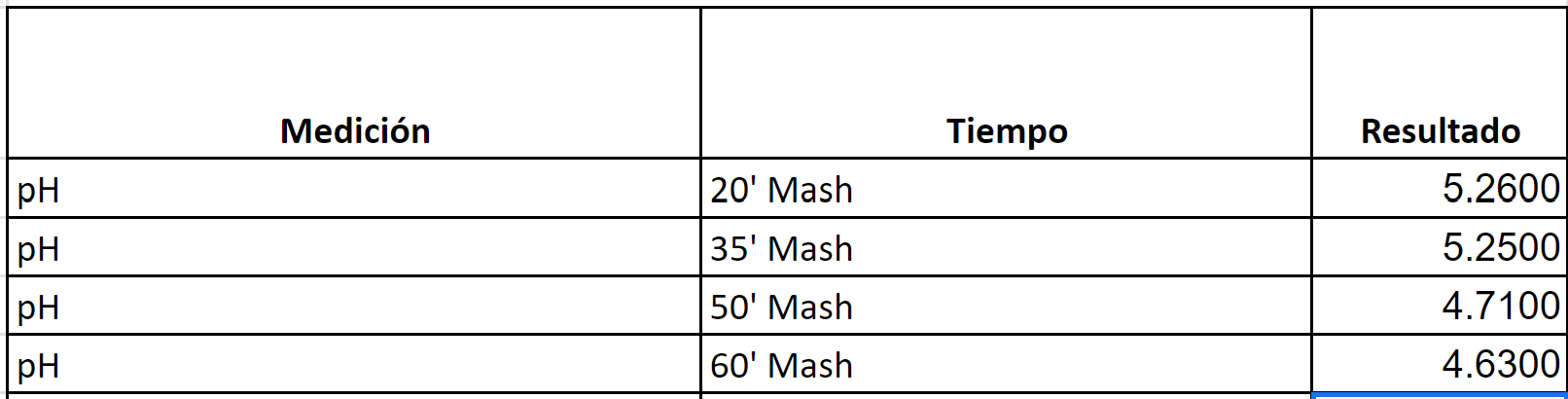
RED IPA (Brewed twice)
Target Mash PH per BrunWater with salt adjustments: 5.2
Water to Grist L/Kg 2.6
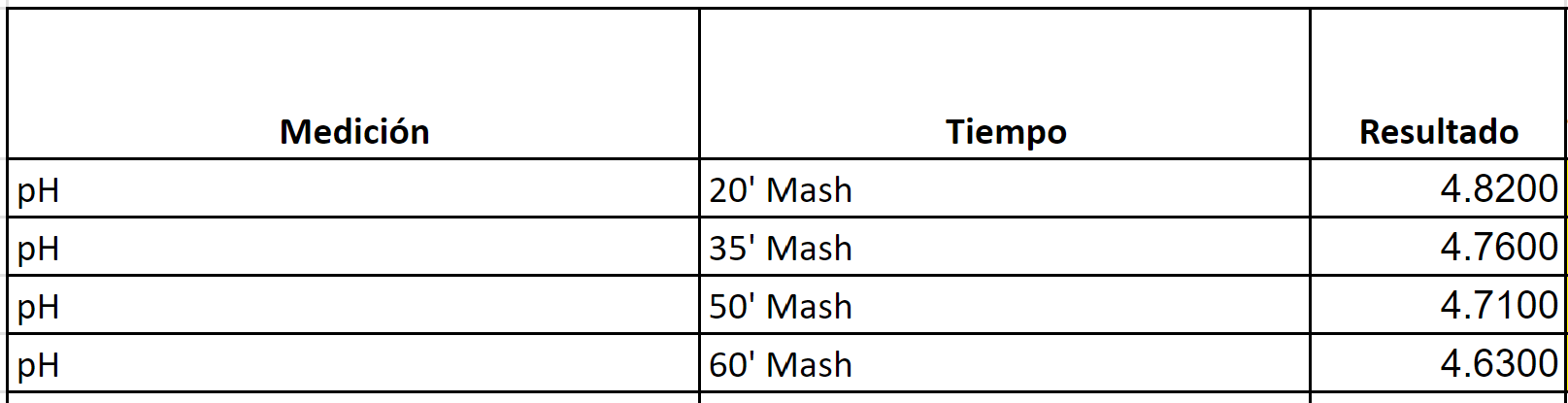
NEIPA
Target Mash PH per BrunWater with salt adjustments: 5.2
Water to Grist L/Kg 2.6
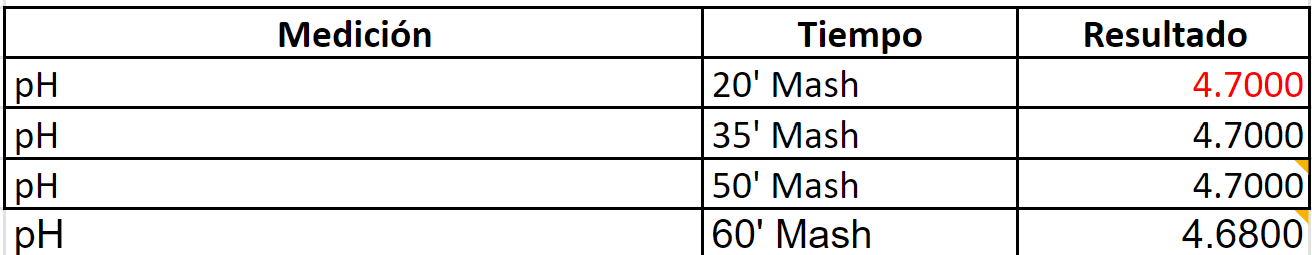
STOUT
Target Mash PH per BrunWater with salt adjustments: 5.18
After 6.9 gr of baking soda to up the Ph
Water to Grist L/Kg 3.1
I will begin by stating that other than not hitting the FG in most of these beers by about 5 points (which could be caused by a number of variables), the beers have all tasted good. I just don't understand why the Ph readings are so far off. Below are different beers, target Ph per BrunWater and actual readings.
I appreciate any insight, what could be the cause, how can I get more consistent in hitting the 5.2-5.4 range.
Thank you!!
Batch Sizes ~30 liters
Water Profile Ph 6.9-7.1
Samples are collected at the indicated times, readings usually take place during the boil, about 60 mins after when all samples have cooled down. I've include a picture of the PhMeter, not a high quality device, but when reading the control substance a chemist friend prepares, it is within 0.1 from the control figure.
RED IPA (Brewed twice)
Target Mash PH per BrunWater with salt adjustments: 5.2
Water to Grist L/Kg 2.6
NEIPA
Target Mash PH per BrunWater with salt adjustments: 5.2
Water to Grist L/Kg 2.6
STOUT
Target Mash PH per BrunWater with salt adjustments: 5.18
After 6.9 gr of baking soda to up the Ph
Water to Grist L/Kg 3.1









