I’ve been using Brewers Friend for over a year now and am still not clear on what’s happening with ion calculations in the Water Chemistry Calculator despite reading the explanation section many times. My basic question is: under the Salt Additions section, what volumes do the total amount of additions apply to when either checking or clearing the box for “Salts Added to Mash Only”? I would expect that if I check this box, the salts I’ve added would apply only to my mash water volume, yet I’m confused by the ion calculation results.
The two attached photos summarize the root of my question. The only change made between these two photos is checking or clearing of the “Salts Added to Mash Only.”. When checked, I’d expect that the key ions that I’ve tried to increase (eg. Ca++, Na+, Cl-, SO4-2) with salt additions would increase since the same quantity of salt additions applies to only the mash water volume rather than the total water volume, yet the opposite result comes back. Further, the mash pH does not change between the two.
Any help would be appreciated. Thanks.
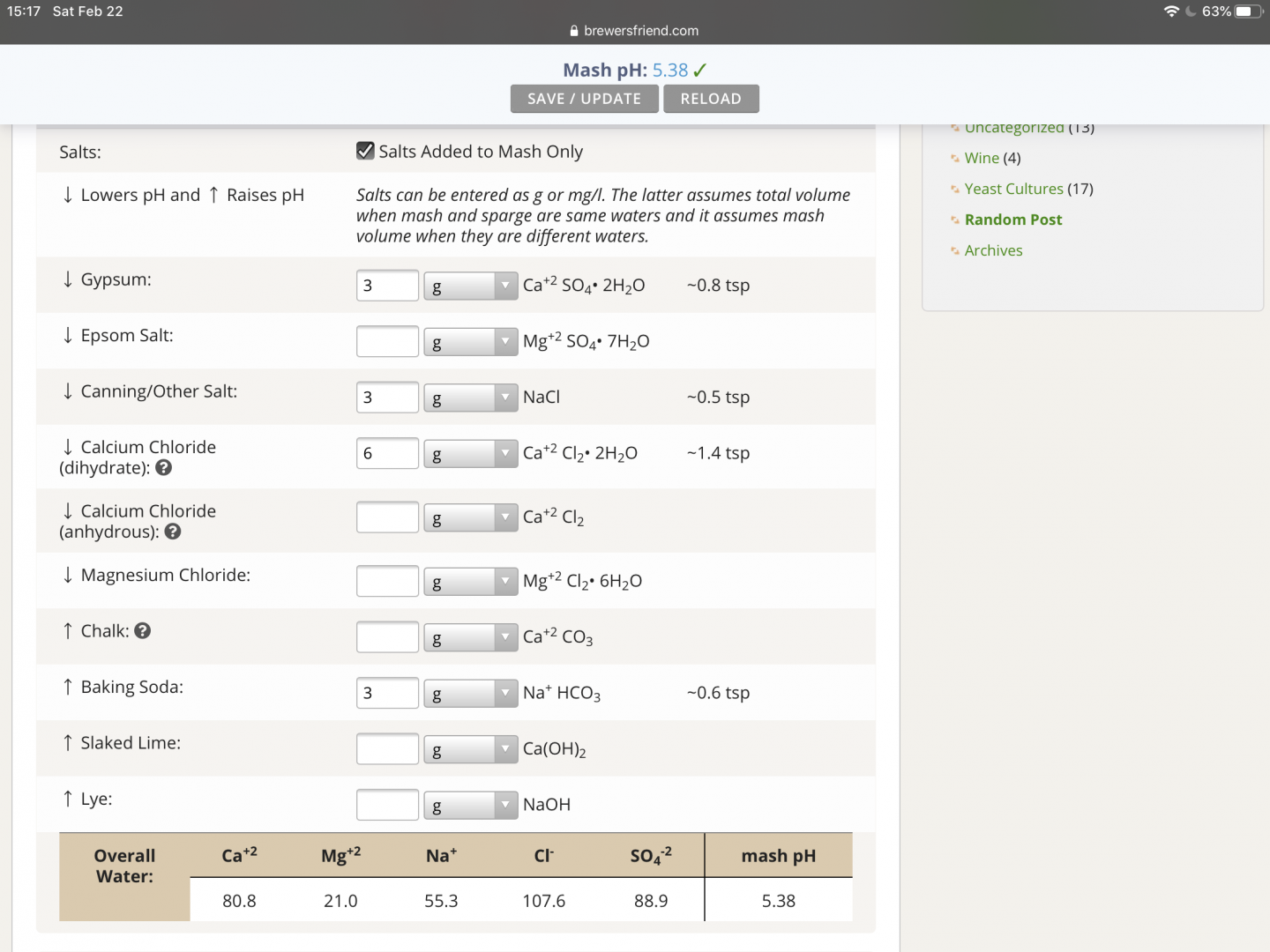
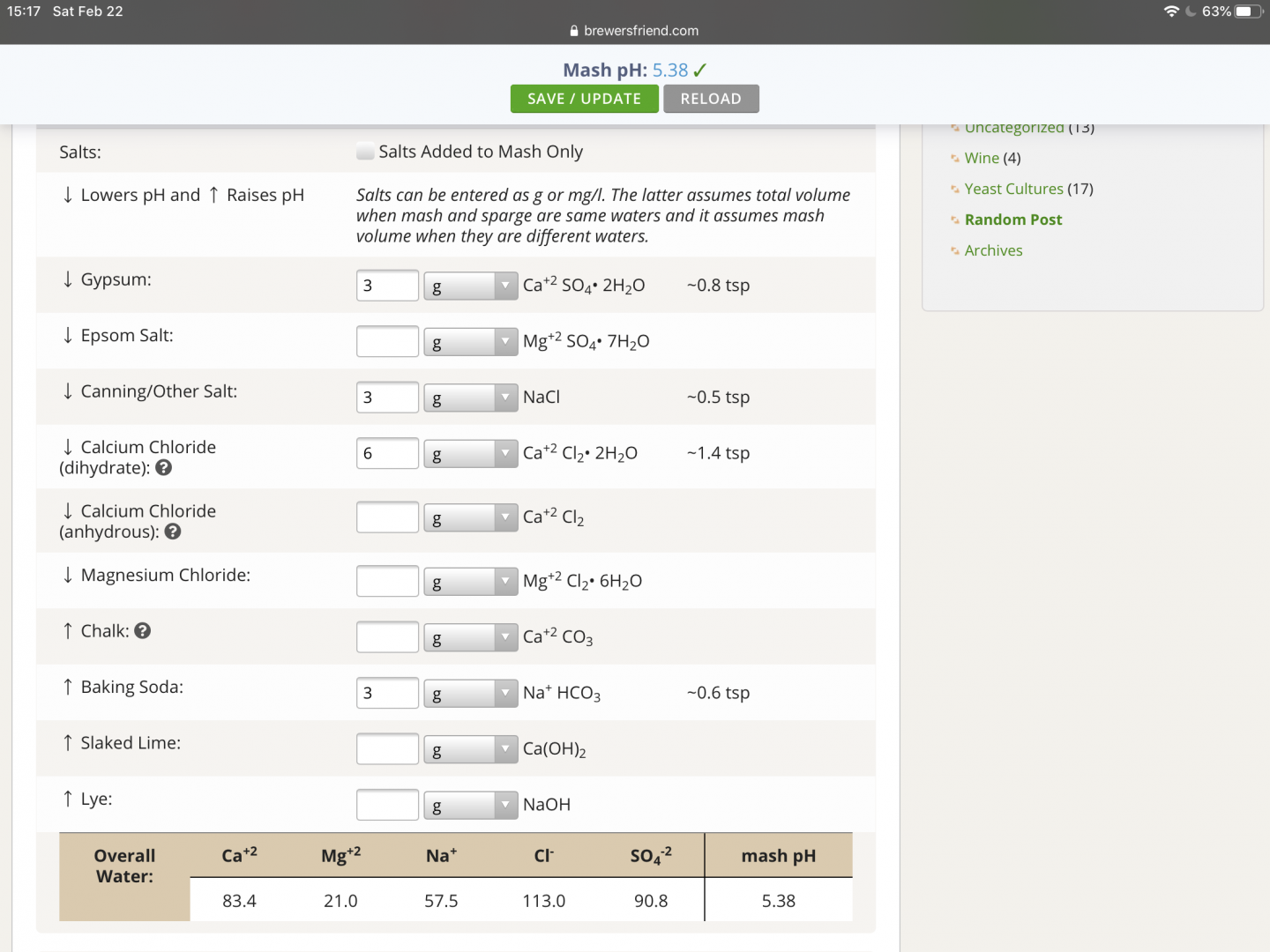
The two attached photos summarize the root of my question. The only change made between these two photos is checking or clearing of the “Salts Added to Mash Only.”. When checked, I’d expect that the key ions that I’ve tried to increase (eg. Ca++, Na+, Cl-, SO4-2) with salt additions would increase since the same quantity of salt additions applies to only the mash water volume rather than the total water volume, yet the opposite result comes back. Further, the mash pH does not change between the two.
Any help would be appreciated. Thanks.









