- Joined
- Feb 17, 2017
- Messages
- 273
- Reaction score
- 183
- Points
- 43
Hello
Attached is water report from my water supply company I found online. I am thinking there is a conversion somewhere to go from mg/L CaCO3 to calcium ions. I have read somewhere that 50 mg/L CaCO3 is approx. 20 ppm Ca2+
Does someone have a good link to send me to for that? I am no where near a chemist, but interested enough to start this step in brewing!
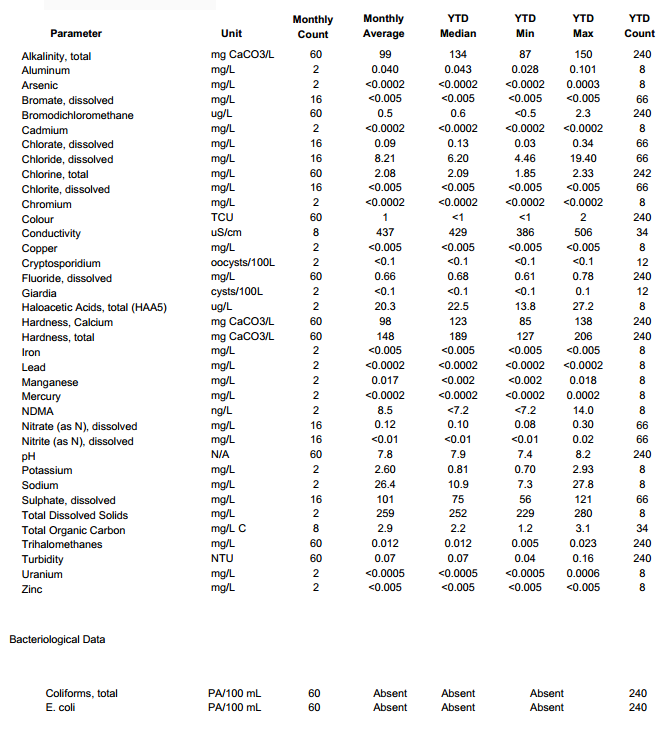
Attached is water report from my water supply company I found online. I am thinking there is a conversion somewhere to go from mg/L CaCO3 to calcium ions. I have read somewhere that 50 mg/L CaCO3 is approx. 20 ppm Ca2+
Does someone have a good link to send me to for that? I am no where near a chemist, but interested enough to start this step in brewing!