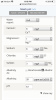
I hate to admit it, but I agree with Gordon Strong’s approach to water. Mostly RO water and simple water salt additions.
The grain brings a lot of its own minerals to the beer like calcium, chlorides and magnesium. The ratio between chlorides and sulfates has been called into question due to the amount of chlorides brought by the malt, which is an unknown quantity to the brewer. It takes a rather large ratio for sulfates to make an impact. I typically use a 4/1 ratio for a German Pils to get the sulfate to dry the beer a bit and bring a more pronounced bitterness.
Water treatments can be kept simple. Blending is also a great option. Just keep in mind that water salts need to be added to help keep the waterborne calcium above 60-70ppm.
Ooof, 4:1? Not that I'm doubting you, but where did you find this information on the contribution of minerals from malt? I'm interested to look into this since I want to brew some nice English bitters with an appropriate hop bite. I was calculating mineral contributions with the SO4/Ch ratio of around 2, but maybe that won't be enough. What kind of concentration of SO4 are you using? And then how do you calculate the Ch level contribution from malt?
BTW, you're just up the river from me, I live in the Twin Cities, so I get my source water from the Mississippi. You must as well. I thought it was pretty good brewing water (but definitely needed some Ca based on the water works reports I get), but have toyed with going to RO or distilled. I imagine the first step would be to brew a beer using RO or distilled water and observing the difference when brewing with municipal water. My sense is that Gordon Strong used RO because his source water in Ohio was just too far out of whack for beer. I wonder what he'd say about our water.