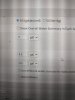
Hello,
I treat all of my mash and sparge water at the same time, in one big kettle, with a focus on getting the mash ph in range. I notice when I do this that the predicted sparge ph is sometimes really low, like 3.7. Is this cause for concern? I know the ideal sparge ph is below 6, but I can't find anyone who says there's a floor.
OR am I doing something wrong with the calculator?
I am working on a recipe right now that is mostly pilsner. To get my mash ph to 5.3, the calculator says I need to add 11 mls of lactic. This results in the sparge ph being 3.6.
My beers turn out fine, but I am just really curious if I a doing something wrong in the calculator. OR if maybe there's nothing to worry about with a low ph sparge. Maybe it all mixes together in the boil kettle...
I treat all of my mash and sparge water at the same time, in one big kettle, with a focus on getting the mash ph in range. I notice when I do this that the predicted sparge ph is sometimes really low, like 3.7. Is this cause for concern? I know the ideal sparge ph is below 6, but I can't find anyone who says there's a floor.
OR am I doing something wrong with the calculator?
I am working on a recipe right now that is mostly pilsner. To get my mash ph to 5.3, the calculator says I need to add 11 mls of lactic. This results in the sparge ph being 3.6.
My beers turn out fine, but I am just really curious if I a doing something wrong in the calculator. OR if maybe there's nothing to worry about with a low ph sparge. Maybe it all mixes together in the boil kettle...