- Joined
- Jun 13, 2017
- Messages
- 722
- Reaction score
- 1,082
- Points
- 93
Ok I want to learn a bit more about how the water calculator works. I have my towns water profile(way down below) setup on this site (did I input it right?) and bring up the water calculator page via the recipe I have, with a target water profile.
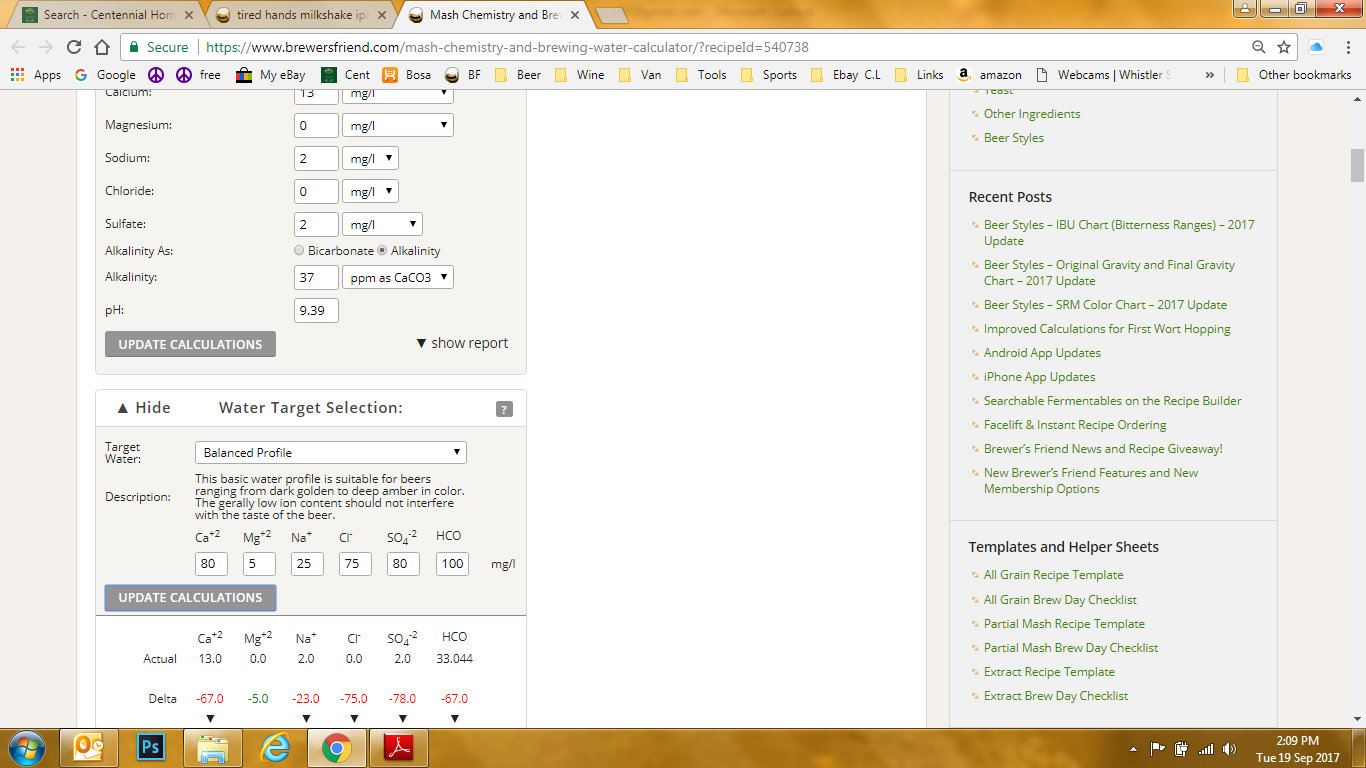
Update the calculations and get this.............now what? What dose all the Delta #'s mean?
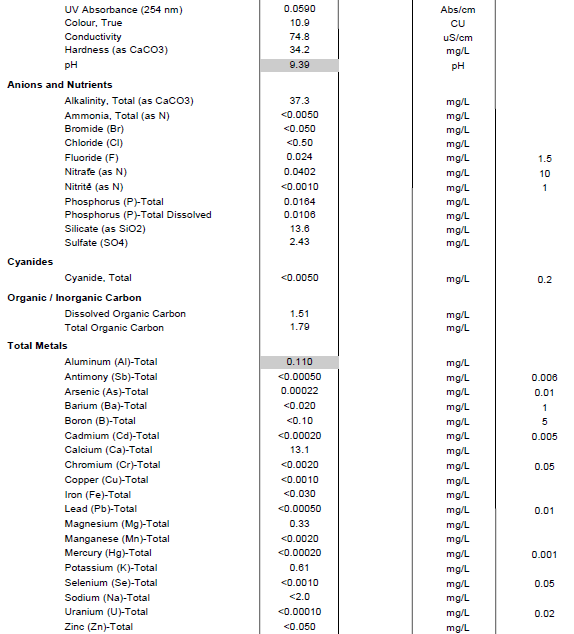
This is my water profile
Update the calculations and get this.............now what? What dose all the Delta #'s mean?
This is my water profile







